Most of the chronometric
dating methods in use today are radiometric
![]() . That
is to say, they are based on knowledge of the rate at which certain radioactive isotopes
within dating samples decay or the rate of other cumulative changes in atoms resulting from radioactivity.
Isotopes
. That
is to say, they are based on knowledge of the rate at which certain radioactive isotopes
within dating samples decay or the rate of other cumulative changes in atoms resulting from radioactivity.
Isotopes
![]() are specific forms of elements. The various isotopes of the same element
differ in terms of atomic mass but have the same
atomic number.
In other words, they differ in the number of neutrons in their nuclei but
have the same number of protons.
are specific forms of elements. The various isotopes of the same element
differ in terms of atomic mass but have the same
atomic number.
In other words, they differ in the number of neutrons in their nuclei but
have the same number of protons.
The spontaneous decay of radioactive elements occurs at different rates, depending on the specific isotope. These rates are stated in terms of half-lives. One half-life is the amount of time required for ½ of the original atoms in a sample to decay. Over the second half-life, ½ of the atoms remaining decay, which leaves ¼ of the original quantity, and so on. In other words, the change in numbers of atoms follows a geometric scale as illustrated by the graph below.
| The red
curve line shows the geometric rate of atomic decay |
 |
The decay of atomic nuclei provides us with a reliable clock that is unaffected by normal forces in nature. The rate will not be changed by intense heat, cold, pressure, or moisture.
Radiocarbon
Dating
The most commonly used
radiometric dating method is radiocarbon
![]() dating.
It is also called carbon-14 and
C-14 dating. This technique is used to date
the remains of organic materials. Dating samples are usually charcoal, wood, bone, or shell, but
any tissue that was ever alive can be dated.
dating.
It is also called carbon-14 and
C-14 dating. This technique is used to date
the remains of organic materials. Dating samples are usually charcoal, wood, bone, or shell, but
any tissue that was ever alive can be dated.
Radiocarbon dating is based
on the fact that cosmic radiation from space constantly bombards our
planet. As cosmic rays pass
through the atmosphere, they occasionally collide with gas atoms resulting in the release of neutrons. When the nucleus of a nitrogen
(14N)
atom
in the atmosphere
captures one of these neutrons, the atom
subsequently changes into
carbon-14
(14C)
after the release of a proton.
The carbon-14 quickly bonds
chemically with atmospheric
oxygen to form carbon dioxide gas.
Carbon-14
is a rare, unstable form of carbon. Only
one in a trillion carbon atoms in the atmosphere is carbon-14.
The majority are carbon-12 (98.99%) and carbon-13 (1.1%).
From a chemical standpoint, all of these isotopes of carbon behave exactly
the same. Carbon dioxide in the atmosphere
drifts down to the earth's surface where much of it is taken in by green
growing plants, and the carbon is used to build new cells by photosynthesis
![]() . Animals eat plants or other animals that have
eaten them. Through this process, a small amount of
carbon-14 spreads through all living things
and is incorporated into their proteins and other organic molecules.
. Animals eat plants or other animals that have
eaten them. Through this process, a small amount of
carbon-14 spreads through all living things
and is incorporated into their proteins and other organic molecules.
|
Natural production |
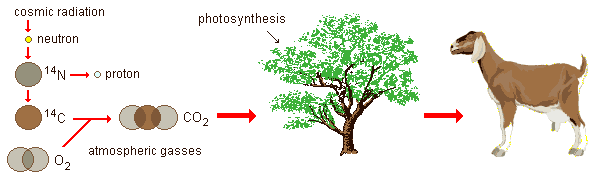 |
As long as an organism is alive, it takes in carbon-14 and the other carbon isotopes in the same ratio as exists in the atmosphere. Following death, however, no new carbon is consumed. Progressively through time, the carbon-14 atoms decay and once again become nitrogen-14. As a result, there is a changing ratio of carbon-14 to the more atomically stable carbon-12 and carbon-13 in the dead tissue. That rate of change is determined by the half-life of carbon-14, which is 5730 ± 40 years. Because of this relatively rapid half-life, there is only about 3% of the original carbon-14 in a sample remaining after 30,000 years. Beyond 40-50,000 years, there usually is not enough left to measure with conventional laboratory methods.
|
|
Half-Lives
|
Years Past
|
C-14 Atoms
|
C-12 Atoms
|
||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 1 N | 1 N | |||||
| 1 | 5,730 | 1/2 N | 1 N | |||||
| 2 | 11,460 | 1/4 N | 1 N | |||||
| 3 | 17,190 | 1/8 N | 1 N | |||||
| 4 | 22,920 | 1/16 N | 1 N | |||||
| 5 | 28,650 | 1/32 N | 1 N | |||||
| 6 | 34,380 | 1/64 N | 1 N | |||||
| 7 | 40,110 | 1/128 N | 1 N |
The conventional
radiocarbon dating method involves burning a sample in a closed tube containing oxygen.
The carbon containing gas
that is produced is then cooled to a liquid
state
and placed in a lead shielded box with a sensitive Geiger counter. This
instrument registers the radioactivity of the carbon-14 atoms. Specifically, it
detects the relatively weak beta particles
![]() released when
carbon-14 nuclei
decay. The age of a
sample is determined by the number of decays recorded over a set period of time.
Older samples have less carbon-14 remaining and,
consequentially, less frequent decays.
Knowing the half-life of carbon-14 allows the calculation of a sample's age.
released when
carbon-14 nuclei
decay. The age of a
sample is determined by the number of decays recorded over a set period of time.
Older samples have less carbon-14 remaining and,
consequentially, less frequent decays.
Knowing the half-life of carbon-14 allows the calculation of a sample's age.
 |
|
|
A
radiocarbon sample |
A relatively new variation
of the radiocarbon dating method utilizes an accelerator mass spectrometer
![]() ,
which is a device
usually used by physicists to
measure the abundance of very rare radioactive isotopes.
When used for dating, this AMS method involves actually counting individual carbon-14
atoms. This allows the dating of much older and smaller samples but at a far higher
cost. Although, organic materials as old as 100,000 years potentially can be dated with AMS, dates older than 60,000 years are still rare.
,
which is a device
usually used by physicists to
measure the abundance of very rare radioactive isotopes.
When used for dating, this AMS method involves actually counting individual carbon-14
atoms. This allows the dating of much older and smaller samples but at a far higher
cost. Although, organic materials as old as 100,000 years potentially can be dated with AMS, dates older than 60,000 years are still rare.
 |
|
|
Radiocarbon and tree-ring
date comparisons |
Paleoanthropologists and archaeologists must always be aware of possible radiocarbon sample contamination that could result in inaccurate dates. Such contamination can occur if a sample is exposed to carbon compounds in exhaust gasses produced by factories and motor vehicles burning fossil fuels such as coal or gasoline. The result is radiocarbon dates that are too old. This has been called the Autobahn effect, named after the German high speed roadway system. Archaeologists in that country first noted this source of contamination when samples found near the Autobahn were dated. The effect of global burning of fossil fuels on radiocarbon dates was verified and calibrated by Hans Suess of the University of California, San Diego when he radiocarbon dated bristlecone pine tree growth rings that were of known chronometric ages. Subsequently, it is also called the Suess effect.
Other kinds of sample contamination can cause carbon-14 dates to be too young. This can occur if the sample is impregnated with tobacco smoke or oils from a careless researcher's hands. This is now well known and is easily avoided during excavation.
Still another potential source of error in radiocarbon dating that is adjusted for stems from the assumption that cosmic radiation enters our planet's atmosphere at a constant rate. In fact, the rate changes slightly through time, resulting in varying amounts of carbon-14 being created. This has become known as the de Vries effect because of its discovery by the Dutch physicist Hessel de Vries.
All of these potential sources of error in radiocarbon dating are now well understood and compensating corrections are made so that the dates are reliable.
Potassium-Argon
Dating
There are a number of other
radiometric dating systems in use today that can provide dates for much older sites than
those datable by radiocarbon dating. Potassium-argon
![]() (K-Ar) dating
is one of them. It is based on the fact that potassium-40
(40K)
decays into
the gas argon-40 (40Ar)
and
calcium-40 (40Ca)
at a known rate. The half-life of potassium-40 is
approximately 1.25
billion years. Measurement of the amount of argon-40 in a sample is the basis for
age determination.
(K-Ar) dating
is one of them. It is based on the fact that potassium-40
(40K)
decays into
the gas argon-40 (40Ar)
and
calcium-40 (40Ca)
at a known rate. The half-life of potassium-40 is
approximately 1.25
billion years. Measurement of the amount of argon-40 in a sample is the basis for
age determination.
Dating samples for this technique are geological strata of volcanic origin. While potassium is a very common element in the earth's crust, potassium-40 is a relatively rare isotope of it. However, potassium-40 is usually found in significant amounts in volcanic rock and ash. In addition, any argon that existed prior to the last time the rock was molten will have been driven off by the intense heat. As a result, all of the argon-40 in a volcanic rock sample is assumed to date from that time. When a fossil is sandwiched between two such volcanic deposits, their potassium-argon dates provide a minimum and maximum age. In the example below, the bone must date to sometime between 1.75 and 1.5 million years ago.
| Using the potassium-argon method to date volcanic ash strata above and below a bone sample in order to determine a minimum and a maximum age |
 |
Potassium-argon dates usually have comparatively large statistical plus or minus factors. They can be on the order of plus or minus 1/4 million years for a 2 million year old date. This is still acceptable because these dates help us narrow down the time range for a fossil. The use of additional dating methods at the same site allow us to refine it even more.
NOTE: the plus or minus number following radiometric dates is not an error factor. Rather, it is a probability statement. For instance, a date of 100,000 ± 5,000 years ago means that there is a high probability the date is in the range of 95,000 and 105,000 years ago and most likely is around 100,000. Radiometric dates, like all measurements in science, are close statistical approximations rather than absolutes. This will always be true due to the finite limits of measuring equipment. This does not mean that radiometric dates or any other scientific measurements are unreliable.
Potassium-argon dating has become a valuable tool for human fossil hunters, especially those working in East Africa. Theoretically it can be used for samples that date from the beginning of the earth (4.54 billion years) down to 100,000 years ago or even more recently. Paleoanthropologists use it mostly to date sites in the 1 to 5 million year old range. This is the critical time period during which humans evolved from their ape ancestors.
A relatively new technique related to potassium-argon dating compares the ratios of argon-40 to argon-39 in volcanic rock. This provides more accurate dates for volcanic deposits and allows the use of smaller samples.
Fission
Track Dating
Another
radiometric method that is used for samples from early
human
sites is fission track
![]() dating. This is based on the fact
that a number of crystalline or glass-like minerals, such as obsidian,
mica, and zircon crystals, contain trace
amounts of uranium-238 (238U), which is an unstable isotope. When
atoms of uranium-238
decay, there is a release of energy-charged
alpha particles
dating. This is based on the fact
that a number of crystalline or glass-like minerals, such as obsidian,
mica, and zircon crystals, contain trace
amounts of uranium-238 (238U), which is an unstable isotope. When
atoms of uranium-238
decay, there is a release of energy-charged
alpha particles
![]() which burn narrow fission tracks, or damage trails,
through the glassy material. These can be seen and counted with an optical
microscope.
which burn narrow fission tracks, or damage trails,
through the glassy material. These can be seen and counted with an optical
microscope.
| Fission tracks in obsidian as they would appear with an optical microscope |
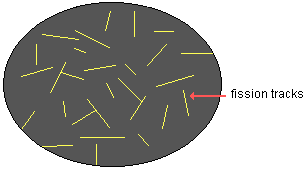 |
The number of fission tracks is directly proportional to the amount of time since the glassy material cooled from a molten state. Since the half-life of uranium-238 is known to be approximately 4.5 billion years, the chronometric age of a sample can be calculated. This dating method can be used with samples that are as young as a few decades to as old as the earth and beyond. However, paleoanthropologists rarely use it to date sites more than several million years old.
With the exception of early
historic human made glass artifacts
![]() , the fission track method is usually only employed to
date geological strata. Artifacts made out of obsidian and mica are not fission track dated because it would only tell
us when the rocks cooled from a molten state, not when they were made into artifacts by our early human
ancestors.
, the fission track method is usually only employed to
date geological strata. Artifacts made out of obsidian and mica are not fission track dated because it would only tell
us when the rocks cooled from a molten state, not when they were made into artifacts by our early human
ancestors.
Thermoluminescence
Dating
Thermoluminescence
![]() (TL) dating is a radiometric
method based on the fact that trace amounts of radioactive atoms, such as
uranium and thorium, in some kinds of rock, soil, and clay produce constant low amounts of background ionizing
radiation. The atoms of crystalline solids, such as pottery and rock, can be altered
by this radiation. Specifically, the electrons of quartz, feldspar, diamond, or
calcite crystals can become displaced from their
normal positions in atoms and trapped in imperfections in the
crystal lattice
(TL) dating is a radiometric
method based on the fact that trace amounts of radioactive atoms, such as
uranium and thorium, in some kinds of rock, soil, and clay produce constant low amounts of background ionizing
radiation. The atoms of crystalline solids, such as pottery and rock, can be altered
by this radiation. Specifically, the electrons of quartz, feldspar, diamond, or
calcite crystals can become displaced from their
normal positions in atoms and trapped in imperfections in the
crystal lattice
![]() of the
rock or clay molecules.
These energy charged electrons progressively accumulate over time. When a sample is
heated to high temperatures in a laboratory, the trapped electrons are released
and return to their normal positions in their atoms. This
causes them to give off their stored energy in the form of light impulses (photons).
This light is referred to as thermoluminescence
(literally "heat light"). A similar effect can be
brought about by stimulating the sample with infrared light. The intensity of
thermoluminescence is directly related to the amount of accumulated changes produced by
background radiation, which, in turn, varies with the age of the sample and the amount of
trace radioactive elements it contains.
of the
rock or clay molecules.
These energy charged electrons progressively accumulate over time. When a sample is
heated to high temperatures in a laboratory, the trapped electrons are released
and return to their normal positions in their atoms. This
causes them to give off their stored energy in the form of light impulses (photons).
This light is referred to as thermoluminescence
(literally "heat light"). A similar effect can be
brought about by stimulating the sample with infrared light. The intensity of
thermoluminescence is directly related to the amount of accumulated changes produced by
background radiation, which, in turn, varies with the age of the sample and the amount of
trace radioactive elements it contains.
| A ground up sample is placed in a special oven |
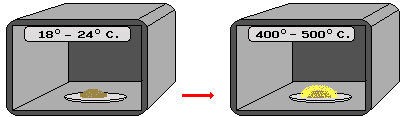 |
Heat is raised rapidly resulting in an energy emission from the sample |
||
|
|
||||
What is actually determined is the amount of elapsed time since the sample had previously been exposed to high temperatures. In the case of a pottery vessel, usually it is the time since it was fired in a kiln. For the clay or rock lining of a hearth or oven, it is the time since the last intense fire burned there. For burned flint, it is the time since it had been heated in a fire to improve its flaking qualities for stone tool making.
The effective time range for TL dating is from a few decades back to about 300,000 years, but it is most often used to date things from the last 100,000 years. Theoretically, this technique could date samples as old as the solar system if we could find them. However, the accuracy of TL dating is generally lower than most other radiometric techniques.
Electron
Spin Resonance Dating
Another relatively new
radiometric dating method related to thermoluminescence is
electron spin resonance
![]() (ESR). It is
also based on the fact that
background radiation causes electrons to dislodge from their
normal positions in atoms and become trapped in
the crystalline lattice of the material. When odd numbers of electrons are
separated, there is a measurable change in the magnetic field
(or spin) of the atoms. Since
the magnetic field progressively changes with time in a predictable way
as a result of this process, it provides
another atomic clock, or calendar, that can be used for dating purposes.
Unlike thermoluminescence dating, however, the sample is not destroyed with the ESR
method. This allows samples to be dated more than once. ESR
is used mostly to date calcium carbonate in limestone, coral, fossil teeth,
mollusks, and egg shells. It also can date
quartz and flint.
Paleoanthropologists have used ESR mostly to date samples
from the last 300,000 years. However, it potentially could be used for
much older samples.
(ESR). It is
also based on the fact that
background radiation causes electrons to dislodge from their
normal positions in atoms and become trapped in
the crystalline lattice of the material. When odd numbers of electrons are
separated, there is a measurable change in the magnetic field
(or spin) of the atoms. Since
the magnetic field progressively changes with time in a predictable way
as a result of this process, it provides
another atomic clock, or calendar, that can be used for dating purposes.
Unlike thermoluminescence dating, however, the sample is not destroyed with the ESR
method. This allows samples to be dated more than once. ESR
is used mostly to date calcium carbonate in limestone, coral, fossil teeth,
mollusks, and egg shells. It also can date
quartz and flint.
Paleoanthropologists have used ESR mostly to date samples
from the last 300,000 years. However, it potentially could be used for
much older samples.
Comparison
of the Time Ranges for Dating
Methods
Whenever possible, paleoanthropologists collect as many dating samples from an ancient human occupation site as possible and employ a variety of chronometric dating methods. In this way, the confidence level of the dating is significantly increased. The methods that are used depend on the presumed age of the site from which they were excavated. For instance, if a site is believed to be over 100,000 years old, dendrochronology and radiocarbon dating could not be used. However, potassium-argon, fission track, amino acid racemization, thermoluminescence, electron spin resonance, and paleomagnetic dating methods would be considered.
|
EFFECTIVE
TIME RANGE OF THE MAJOR
CHRONOMETRIC DATING METHODS |
|
 |
In addition to the likely time range, paleoanthropologists must select dating techniques based on the kinds of datable materials available. Dendrochronology can only date tree-rings. Any organic substances can be used for radiocarbon and amino acid racemization dating. Calcium rich parts of animals such as coral, bones, teeth, mollusks, and egg shells can be dated with the electron spin resonance technique. In addition, ESR can date some non-organic minerals including limestone, quartz, and flint. Burned clay and volcanic deposits are materials used for paleomagnetic dating. Glassy minerals, such as mica, obsidian, and zircon crystals are datable with the fission track method. Pottery and other similar materials containing crystalline solids are usually dated with the thermoluminescence technique. The potassium-argon and argon-argon methods are used to date volcanic rock and ash deposits.
Other chronometric dating methods not described here include uranium/thorium dating, oxidizable carbon ratio (OCR) dating, optically stimulated luminescence (OSL) dating, varve analysis, and obsidian hydration dating.